Electromechanical Systems Power Density Capability Electromagnetic motors generators and actuators are limited by magnetic field saturation and can produce up to about 200 pounds per square inch of actuator. This is adiabatic compression.
260h Licensed For Non Commercial Use Only Expansion And Compression Work

Thermodynamics Processes

Assertion Isothermal And Adiabatic Two Processes Are Shown On P
Reversible irreversible adiabatic processes.

Adiabatic vs isothermal. An adiabatic process is defined as one of the thermodynamic processes which occur without any heat transfer between the system and the surrounding. As a key concept in thermodynamics the. X or use some numerical technique to find V.
The isentropic head is calculated by equation 3A. In hydraulic systems 3000 to 8000 pounds per square. Calculating Final Temperature and Volume of Adiabatic Expansion.
Here P Pressure Nm2 T Temperature K. For isothermal compression of an ideal gas the PV graph is a concave-up hyperbola called an. Adiabatic or non-isothermal exothermic reactions Product inhibited reactions some enzymes Series of Reactors Example.
Quasi-staticmeans slow enough that the system is always near thermal equilibrium. This material quality is very important for structural and machine parts to endure shock and vibrationSome examples of tough material are manganese wrought iron and mild. Energy flow in an air conditioner or fridge.
A longitudinal wave on a spring. An ideal isothermal process must occur very slowly to keep the gas temperature constant. In a sense isothermal process can be considered as the opposite extreme of adiabatic process.
A thermodynamic process which occurs at constant temperature is known as isothermal process. A thermodynamic process in which there is no heat transfer involved is called adiabatic process. Is ΔG the maximum non expansion work for reversible processes only.
313 are compared it can be seen that pressure reduction during gas expansion is more significant in the adiabatic process than in the isothermal process since no heat is transferred from the surroundings to the gas enclosed in the cylinder. The thermodynamic process is where the movement of heat energy takes place either within an objectarea or between objectsareas. Thus in an isothermal process the internal energy of an ideal gas is constant.
What is Toughness. Adiabatic and isothermal quasi-static processes are reversible because there is no heat flow from hot to cold. A wave movie and a graph.
Irreversible process Isothermal and Adiabatic 4. In thermodynamics an adiabatic process Greek. Isothermal isobaric and isochoric processes.
2 CSTRs F Ao x 2 v 1 v 2 A1 F x 1 A2 F Figure 3. Isothermal isometric adiabatic processes Opens a modal Second law of thermodynamics Opens a modal Practice. The term dry implies to parcels of air without water content.
Equations for a pressure drop of adiabatic flow. Latent Heat vs Sensible Heat When the energy of a system changes because of a temperature difference between the system and its surroundings we say that energy has been transferred as heat q. For example if an ideal gas makes a quasi-static adiabatic transition from a state with pressure and volume and to a state with and then it must be true that.
Heat transfer takes place from high temperature to low temperature which is according to a temperature gradient. The curves are approximately. Generally compressors have performance characteristics analogous to pumps see Chapter 5 of Vol.
Adiábatos impassable is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environmentUnlike an isothermal process an adiabatic process transfers energy to the surroundings only as work. Learn about the isobaric isochoric isothermal and adiabatic. C A chemical reaction takes place in a closed adiabatic perfectly insulated rigid container.
Moist or saturated adiabatic lapse rate and the dry adiabatic lapse rate are the two types of lapse rates. A sample thermodynamic cycle. The PV curve for adiabatic compression called an adiabat begins on a lower-temperatureisothermand ends on a higher.
The pressure drop of adiabatic flow can be calculated using the following equations. If heat is added or taken away during the compression process this is called isothermal compression. Energy flow in a heat engine.
F V Ao 11 X rA 1 2nd reactor. And based on the polytropic process. The purpose of a compressor is to convert shaft work into a useful output that is air flow.
In isothermal processes heat exchange is slow enough so that the systems temperature remains constant. If the p-v diagrams for adiabatic and isothermal processes in Fig. This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process.
The square root of the absolute temperature. The isothermal process however is seldom used as a basis because the normal industrial compression process is not even approximately carried out at constant temperature. This is always true not just for ideal gases.
Hydraulic Pneumatic Systems vs. The dry adiabatic lapse rate is simply unsaturated. Well discuss this more later.
Even if the piston-cylinder device is covered with thermal insulation. In practice most expansion and compression processes are somewhere in between or said to be polytropic. Change in water vapor with change in pressure and volume.
Comparing isothermal and adiabatic processes. Phase diagrams Opens a modal Enthalpy Opens a modal Heat of formation Opens a modal. This is a consequence of Joules second law which states that the internal energy of a fixed amount of an ideal gas depends only on its temperature.
Levenspiel Plot for an exothermic adiabatic reaction. Actual compression processes are polytropic because the gas being compressed is not at constant entropy as in the adiabatic process or at constant temperature as in the isothermal processes. PV diagrams - part 2.
For High-Pressure drop service in ordinary gas piping the flow is closer to adiabatic than truly isothermal. The adiabatic condition of can be written in terms of other pairs of thermodynamic variables by combining it with the ideal. 1 4 th Ed.
Toughness is a measure of how much deformation a material can undergo before fractureIn other words it is the ability to withstand both plastic and elastic deformations. As compressing air generates heat all of the heat is retained within the compression chamber. For an isentropic reversible and adiabatic process equation 1 can be written as.
D Repeat part c only suppose the reactor is isothermal rather than adiabatic and that when the reaction was carried out adiabatically the temperature in the reactor increased. An isothermal process is defined as one of the thermodynamic processes which occur at a constant temperature. The observation that the pressure of an ideal gas is inversely proportional to its volume at constant temperature.
Schematic of two CSTRs in series. We can now use the techniques developed in Chapter 2 to size reactors and reactors in series to compare and size CSTRs and PFRs. Isothermal processes are of special interest for ideal gases.
An ideal adiabatic process must occur very rapidly without any flow of energy in or out of the system. The polytropic process can be expressed as. Difference Between Moist and Adiabatic Rates MOIST vs DRY ADIABATIC RATES Lapse rates imply warming and cooling of air.
Why an adiabatic process changes temperature. When finished plot vs.

Work Done In Isothermal Vs Adiabatic Process Physics Stack Exchange

Comparison Of Adiabatic And Isothermal Compression 6 Download Scientific Diagram
3 4 Gas Expansion Chemistry Libretexts
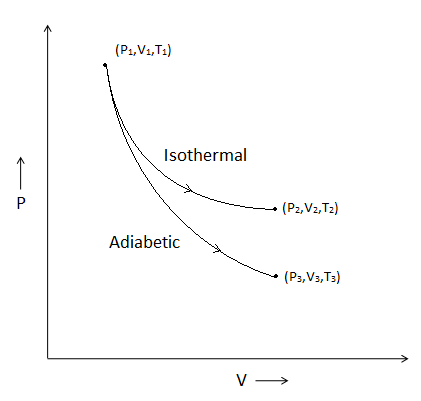
The Reversible Expansion Of An Ideal Gas Under Adiabatic Class 11 Chemistry Cbse
Difference Between Adiabatic Isothermal And Isobaric Difference Between

Adiabatic Vs Isothermal What S The Difference Ask Difference

Isothermal And Adiabatic Two Processes Are Shown On P V Diagram Process 1 Is Adiabatic And Process 2 Is Isothermal

Difference Between Adiabatic And Isothermal Compare The Difference Between Similar Terms
